Iron (Fe) is an essential player in the development and vitality of plants, playing a fundamental role in processes as important as photosynthesis, the formation of chlorophyll, and resistance to environmental stress.

Fe in cannabis cultivation
Fe isn’t a mobile element and isn’t part of chlorophyll, but it does contribute to the pigmentation and respiration of cannabis plant leaves. It also directly interacts with the production of enzymes.
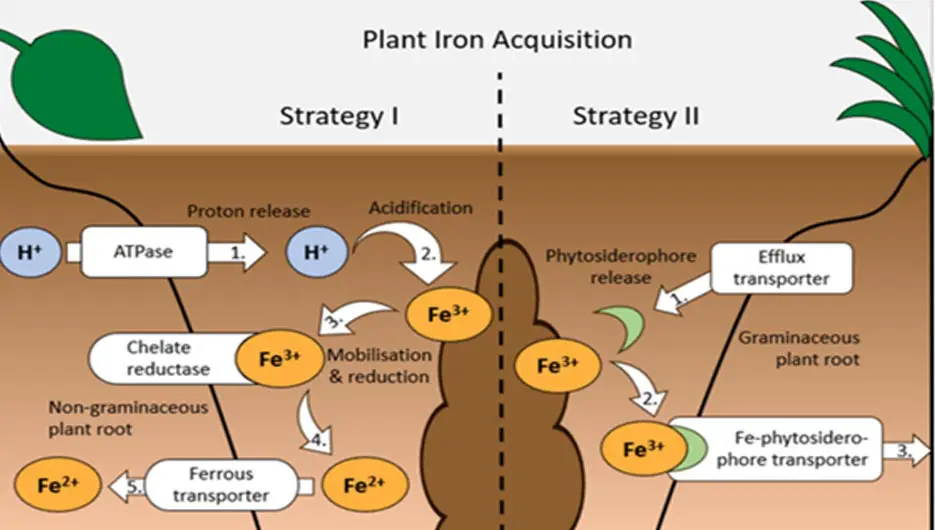
The plant can assimilate it in the form of Ferric ion (Iron ion with a positive charge of +3, Fe3+) or in its organic form.
It is an inorganic compound that belongs to the class of homogeneous transition metal compounds.
Plants use different mechanisms to absorb the Fe in the substrate, especially when it comes to formats that are difficult for them to assimilate. Through the chelation mechanism (a chemical reaction that occurs when molecules bind to metal atoms; this is used in medicine to eliminate heavy metals from the body), which is based on the creation of siderophores (molecules that are responsible for transporting Fe to microorganisms, produced by bacteria, fungi and some plants) that come into confluence with different bacteria, they will make the micronutrient more usable for Cannabis plants.

Beggining of the deficiency
Types of quelates:
- Fe-EDTA: This chelate is suitable for substrates with a pH below 6.0; at an above pH 6.5, half of the Fe will be unavailable, so it will not be very functional.
- Fe-DTPA: This chelate is known for remaining stable at pH 7.0 and for not displacing Fe with Ca.
- Fe-EDDHA: This chelate remains stable up to pH 11.0 but is also the most expensive.
Iron deficiency in cannabis cultivation
Deficiencie of this element usually stem from a substrate’s pH value above 7,0. At this value the plant can’t absorb Fe through its roots.
Fe is no longer assimilated by the plant even when is present in the substrate in cases such as: high carbonate levels, high salinity, constant humidity, low temperatures or in case of an excess of other microelement that cause Iron deficiency.
In case of deficiency the youngest leaves (the ones at the top of the plant) will suffer chlorosis while maintaining the green veins. In other words; the leaves will gradually discolor while the veins remain green.

Mid-stage Iron deficiency
Causes of deficiency:
- Lack of Fe in the soil (possible but rare)
- Immobilization of Iron in an alkaline substrate which can be caused by an excess of Calcium (Ca) or bicarbonate in the irrigation water.
- The presence of certain organisms that transform Iron into Ferrous oxide which is not absorb by plants
- Interaction with other cations caused by an excess of heavt metals (Mg, Cu, Zn, etc.)
- Weak root system due to poor drainage, low temperatures, lack of oxygen, etc.
- Excessive light intensity.
How to solve the deficiency?
- Reduce the alkalinity of the substrate adding Sulfur (S), horse poop and organic fertilizers along with fertilizers that acidify the substrate.
- Provide the plant with Fe chelates for a fast absorption within an optimal pH range.
- Foliar application of Iron sulfate to overcome a minor deficiency.

Advanced phase of Fe deficiency
Fe Excess in cannabis cultivation
An excess of this micronutrient causes:
- Chlorosis and leaf yellowing: Although Fe is essential for the formation of chlorophyll, an excess could cause chlorosis, manifesting as yellowing of the leaves, especially between the veins.
- Root damage: Fe excess in the soil could damage roots affecting their ability to absorb nutrients and water.
- Reduced growth: Iron excess could interfere with the absorption of other nutrients which could result in decreased plant growth.
- Brown or red spots on leaves: There may be visible accumulation of Fe on the leaf.
- Increased susceptobility to disease: Iron excess could make plants more susceptible to certain pests and diseases.